Table of Contents
Webinar Video
Introduction
My previous blogs have been concerned with our understanding of what happens in a single blood capillary cell. This one deals with how cavernoma are formed, develop and grow.
If you just want to know the answer, jump to section 5H. But the blog also describes how we know the answer – the science behind this understanding. At the end it summarises what we now know and what we don’t yet know about cavernoma development.
Two earlier blogs in the series, “An introduction to cavernoma genetics” and “The two-hit mechanism”, provide important background summarised in 5C.
A cavernoma has many cells, probably many thousand in a large cavernoma. How does a cavernoma develop from this initial event?
- Does a cavernoma originate from a single blood capillary?
- Do all the cells in the cavernoma originate from a single initiating cell?
- Do the cells all have somatic mutations?
- What is the origin of the additional cells in the cavernoma?
- Does a cavernoma automatically develop from that initial trigger?
- What stops a cavernoma growing?
The answers to some of these questions have been answered recently mainly from work by two groups of scientists. The first part of the blog introduces some of the experimental methods used. The second part shows what they discovered.
5A. key Experimental Points
- Since many experiments cannot be done on humans, such research is done on what are termed ‘animal models’. The experiments in this blog were undertaken in mice.
- This research investigates the detailed structure of cavernoma during their formation and development. It does so using a confocal microscope, a special form of light microscope that produces high resolution three-dimensional images.
- The event that initiates the formation of a cavernoma in life is a mutation in a single cell wall of a blood capillary. To mimic this in these laboratory experiments, the normal cavernoma gene is exchanged with a mutant CCM gene created in the laboratory, known as a transgene.
- The transgene incorporates a fluorescent marker so that it lights up in ultraviolet light. Four different such transgenes were created, differing only in the colour of their fluorescent marker (see 5E). None of the other genes carry such a marker. Since the confocal microscope illuminates the cavernoma under UV light, the images show only
cells containing a transgene; other cells don’t show. This tells us about the structure of the developing cavernoma. - The timing of when the transgene is introduced into the cell is set by a trigger chemical so that the experimenter can control when to start cavernoma formation. This is often shortly after birth in the mouse.
5B. Development of Cavernoma in Mice
As in humans, mice have the three cavernoma genes, CCM1, CCM2 and CCM3.
For these experiments ‘familial’ breeds of mice were used in which one copy in each cell is the normal Tight Junction (TJ) form and one copy is the mutant CCM form. This means that only a single mutation is required to enable a cavernoma to form (see 5C).
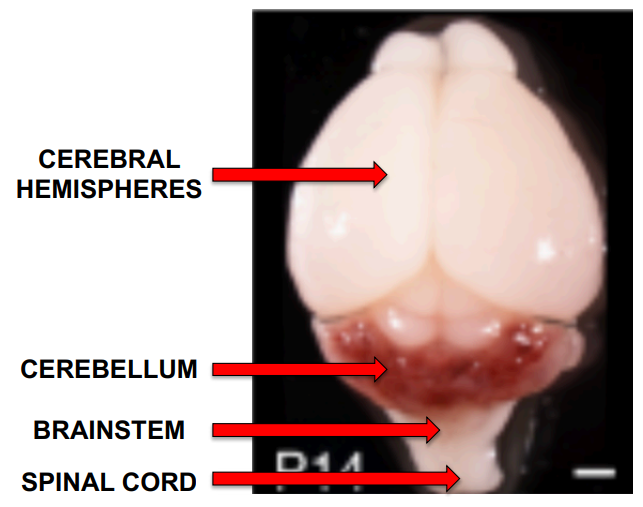
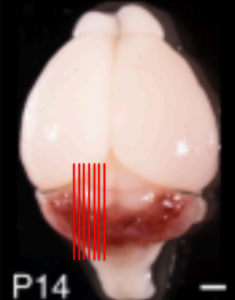
The image on the right is a photograph of a mouse brain 14 days after birth showing the parts of the brain most relevant to cavernoma.
In this mouse image, the cavernomas show up as red, and at this stage of development they are almost exclusively in the cerebellum. Later they also form in the cerebral hemispheres.
Box 2. Nomenclature
It is common to talk about the three cavernoma genes, CCM1, CCM2 and CCM3, being the genes implicated in the formation of cavernoma. It is important to understand that all genes can exist in different/alternative forms, known as alleles, and that the different forms can have very different effects. In these blogs, the letters “CCM” are used for the allele that is responsible for cavernoma formation, found in less than 0.1% of the population; ‘TJ’ is used to refer to the “normal’ form (i.e. in over 99.9% of the population), responsible for the tight junctions between neighbouring blood capillary cells and thus the blood-brain barrier.
5C. Somatic Mutations
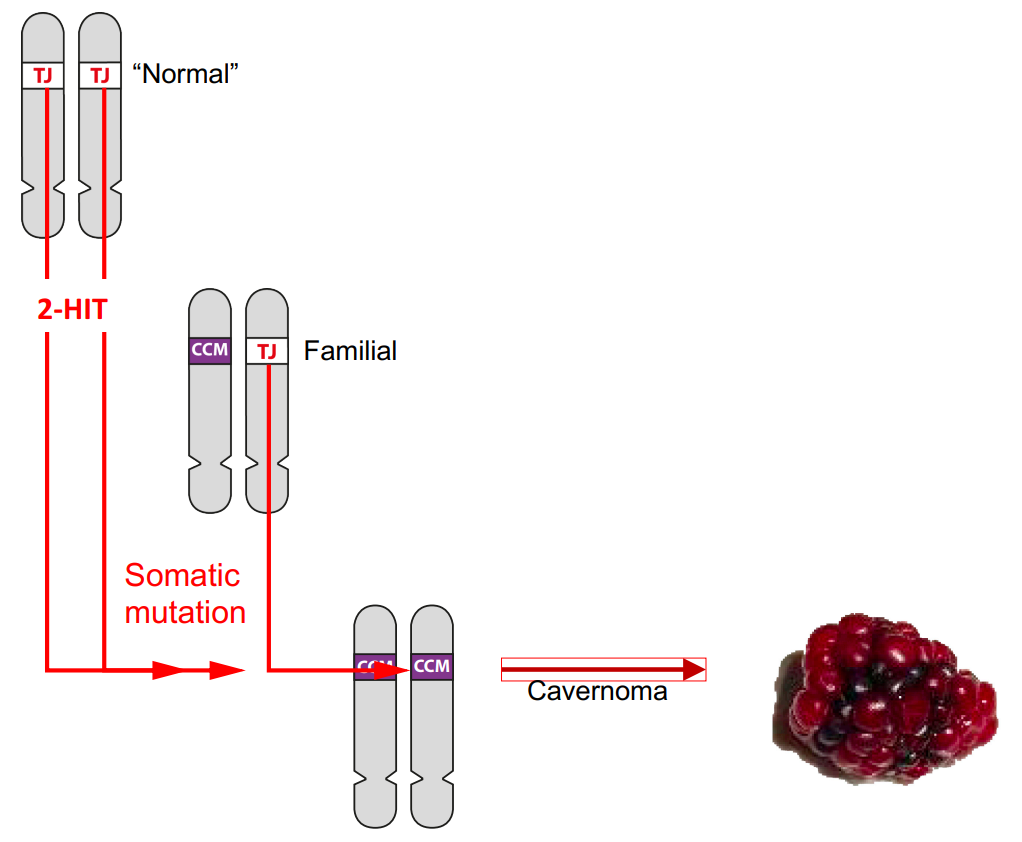
In Blog 1 we learned that there are two forms (alleles) of each of the cavernoma genes. The ‘normal’ Tight Junction (TJ) form is found in over 99.9% of the population, and in most people both copies of the cavernoma gene are the TJ form..
The 0.1% of people with familial cavernoma inherit the mutant CCM form from one of their parents and the normal TJ form from the other. However, in order for a cavernoma to form, both copies of the cavernoma gene must be the mutant CCM form.
This requires a mutation in each TJ gene. Such mutations (hits) are known as ‘somatic’ mutations. They occur during a person’s lifetime in individual cells and are not inherited. This process is described more fully in Blog 2. Two hits are required in a person with two TJ genes,
but just one hit in a person inheriting a mutant CCM gene from one of their parents.
5D. Transgenes
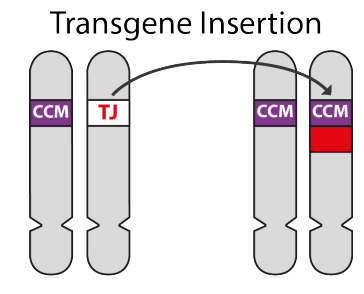
In the laboratory it is now possible to remove a gene and replace it with another. This is known as transgenics, and the replacement is known as a transgene.
In the experiments discussed in this blog, using mice with familial cavernoma (i.e. with one normal TJ gene and one mutant CCM gene), the TJ gene was replaced with a mutant CCM transgene to enable a cavernoma to form.
In addition to the CCM gene, the transgene included an extra piece that forms a fluorescent dye. This means that capillary wall cells with this CCM-Dye transgene will glow under ultraviolet light, enabling the researcher to follow them.
So, what happens?
Capillary cells that have this mutant CCM transgene lose their tight junctions and initiate the formation of a cavernoma. These cells can be identified from their fluorescence, and their role in cavernoma development can be followed in the structures seen with the confocal microscope. The transgene is triggered to replace the TJ gene by injection of a trigger molecule. This means that the timing of when the TJ gene is replaced can be accurately controlled.
5E. Use of different-coloured Transgenes
In these experiments, four different CCM3-dye transgenes were made, each with a different colour fluorescent marker.
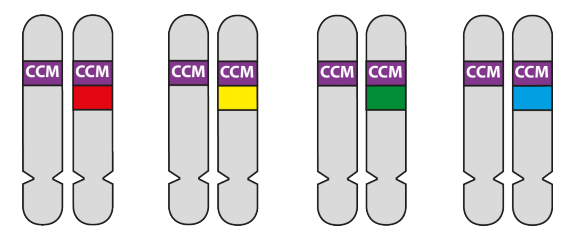
Which colour CCM-dye transgene any individual cell will pick up will be random. The proportion of the capillary cell walls that incorporate the CCM3 gene will depend on the concentration of transgenes injected into the mouse. In the example below, the concentration is low so that only a small fraction of the capillary wall cells received the transgene – the rest were unchanged. At higher concentrations more cells would incorporate CCM transgenes.
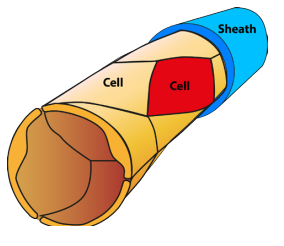
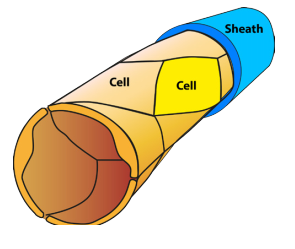
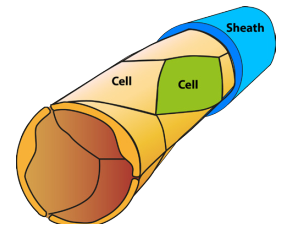

5F. Detailed Structure of the Cavernoma
The detailed structure of cavernoma in the cerebellum was determined by looking at them in sections as indicated in the figures.
The normal way of getting sections is to cut the cerebellum into thin slices and look at these on a slide under a microscope.
A special type of microscope, a confocal microscope, was used in these experiments. A confocal microscope allows a much thicker slice to be cut, and the microscope itself can look at thin slices within the thick one by focussing the microscope up and down.
Computer wizardry then enables three-dimensional diagrams of the structures in those thick slices to be made. The three-dimensional structures are shown in video recordings, some in the webinar that accompanies this blog.
In these experiments the cavernoma and blood capillaries are coloured with fluorescent dyes (described below in 5G) which are stimulated with ultra-violet light. This means that the cavernoma can be seen in detail unencumbered by other material present in the thick slices.
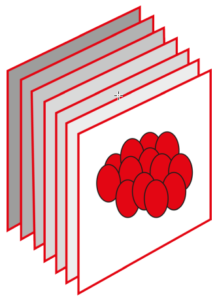
5G. The Results
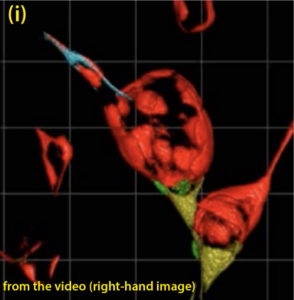
The figure (i) below illustrates an image taken from a video which shows the three-dimensional structure of a cavernoma at a fairly early stage of development. (ii) is a diagram to help understand some significant detail showing just the cavernoma.
Only cells with mutant CCM genes show up. Note that in this case all the cells show the red fluorescence. If you look at the video you will see that, as the image rotates, this cavernoma has the shape of a slightly squashed soccer ball. There are areas on the surface that are transparent (arrow (3)) through which the cells on the far side of the cavernoma can be seen. (1) CCM cells on the top surface of the cavernoma, (2) CCM cells, coloured orange in this diagram seen, through transparent areas, to the far side inside the cavernoma. (4) points to the blood capillary from which this cavernoma has developed. There are at least several hundred cells lining the surface of this cavernoma. Each coloured area contains many identical cells.
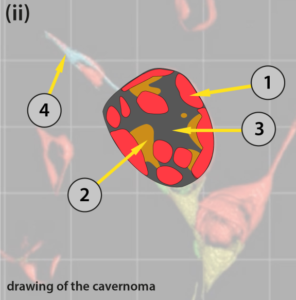
This is just one image. The researchers analysed many images at different stages of cavernoma formation. The significant findings they made were:
- When the cavernomas are small and in the very early stage of development they are entirely formed from cells with a single coloured mutant CCM gene. This shows that these cells are clones of a single initiating cell, otherwise most of the cavernoma would have contained cells of more than one colour.
- Different cavernoma were different colours, each seeded by a different coloured CCM-dye transgene.
- When the cavernoma are larger and later in their formation, as in the diagram above, they are formed of mutant CCM cells of only one colour but with areas in which the cells do not show fluorescence. These areas (the black areas in the images) are of probably cells with a TJ gene.
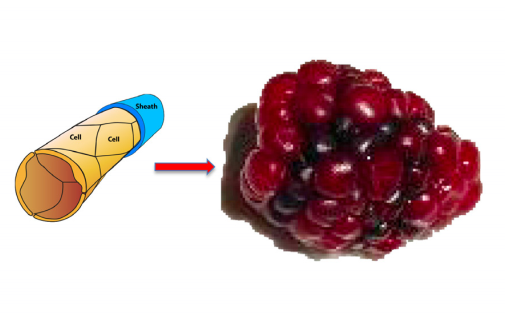
- When the cavernoma develop further and become larger, the cavernoma develop lobes consisting of a mixture of CCM cells and normal TJ cells. This is the origin of the raspberryshaped appearance.
5H. What we have learned
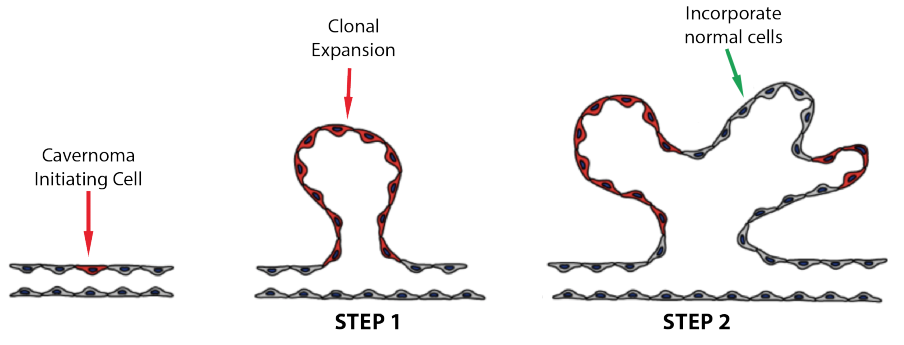
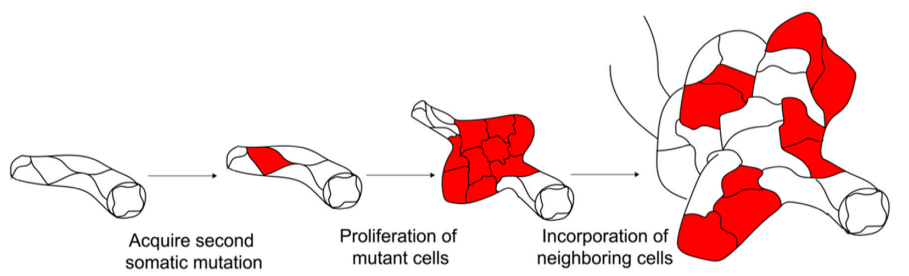
After a single ‘initiating’ cell has a mutant CCM form on both copies of one of the three cavernoma genes, then a cavernoma develops in two steps:
Step 1: That initiating cell duplicates to form many copies (clones) of itself. This is known as ‘clonal expansion’.
Step 2: The developing cavernoma incorporates normal cells (i.e. cells with the TJ form of the genes) between the areas of clonal expansion.
The cavernoma develops the lobed structure resulting in the raspberry appearance of the mature cavernoma. The two diagrams below are taken from papers from two different laboratories (references in the introduction to the blog)
5I. What we know
These experiments have answered many of the important questions to which we didn’t know the answers:
- A cavernoma develops from a single cell on one blood capillary
- The initial development is by clonal expansion of that single cell.
- At a later, but still early stage, normal cells are incorporated into the cavernoma.
References
This blog is heavily based on three fascinating papers:
Detter MR et al (2018): https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.118.313970
Malinverno M et al (2019) https://www.nature.com/articles/s41467-019-10707-x
Abdelilah-Seyfried S et al (2020) https://doi.org/10.1016/j.molmed.2020.03.003